Avian hepatitis-E-virus infection in chickens – Part 2
Avian hepatitis-E-virus infection is a disease of broiler breeders and table-egg layers caused by a hepevirus. This article is the second part of a two-piece description which elaborates on lesions found with necropsy and microscopic examination and the diagnosis of the disease.
The first part of this artice can be found here.
By Dr Tahseen Aziz, Rollins Animal Disease Diagnostic Laboratory and Dr H. John Barnes, College of Veterinary Medicine, NC State University, both in Raleigh, North Carolina, USA
Gross and microscopic lesions have been described in birds with avian hepatitis-E-virus infection.
Hepatitis-splenomegaly syndrome
At necropsy, dead birds are in good body condition. Livers are enlarged, pale, somewhat friable, mottled, stippled with red, yellow, or tan foci, and usually have subcapsular haemorrhages or hematomas, with one or more blood clots loosely adhering to the surface. Small to moderate amounts of blood stained (red) fluid in the abdominal cavity is a common finding. Sometimes a blood clot is found in the abdominal cavity. Spleens are frequently enlarged (mildly to markedly) and their capsular and cut surfaces may show whitish foci. Ovaries are either unremarkable in appearance or regressing.
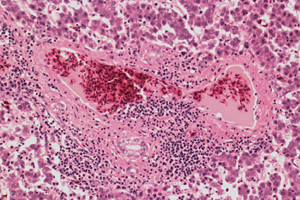
Microscopically, different liver lesions have been described in field cases and in chickens experimentally inoculated with avian HEV isolated from chickens affected with hepatitis-splenomegaly (HS) syndrome. In severe cases, there is multifocal to extensive haemorrhage that markedly disrupts the normal architecture of hepatocellular cords and sinusoids, with replacement of hepatic tissue by pools of erythrocytes. Multifocal to extensive areas of coagulative necrosis of hepatocytes are present and may be infiltrated with lymphocytes.
Portal areas show mild to marked infiltration of both heterophils and mononuclear inflammatory cells and there is often segmental phlebitis and perivenular hepatitis characterised by infiltration of the walls of terminal portal and hepatic venules and perivenular hepatic tissue by mononuclear inflammatory cells. Deposition of amyloid commonly occurs in sinusoids. Areas of hepatic tissue are totally replaced by deposits of acellular or hypocellular, dense, amorphous, homogenous eosinophilic material that does not stain positive for amyloid. Collections of granulocytic myeloid cells have been noted. Caseous granulomas have occurred in some cases. Lesions found in spleens include marked proliferation of splenic reticuloendothelial macrophages (causing enlargement of the spleen), lymphoid depletion, and accumulation of amyloid in the walls of arterioles and interstitial spaces.
Big liver and spleen disease
Birds found dead or showing signs of sickness are usually in good body condition, but the crop is empty, indicating anorexia. Marked enlargement of the spleen (splenomegaly) is most common and may be the only lesion in affected birds. Spleens are usually two to three times normal size and show mottling of the capsular surface and numerous white foci on cut surface. Many birds with splenomegaly also have enlarged livers, which may contain subcapsular haemorrhages. Ovaries are usually regressing, with blood clots frequently present within flaccid yolk follicles.
Microscopically, lesions in the spleen vary with the stage of the disease. Initially, there is a uniform increase in the size of periellipsoidal lymphoblastic regions, which is responsible for the splenomegaly. This is followed by wide spread pyknosis of lymphoid cells in lymphoid follicles, periarteriolar lymphoid sheaths, and other areas of white pulp. This pyknotic destructive phase is followed by a histiocytic response, in which the number of macrophages increases greatly along with occasional mild heterophilic infiltrates. Individual or groups of macrophages show phagocytosis and some appear to undergo necrosis. Areas of necrotic tissue (presumably aggregated necrotic macrophages) may contain nuclear debris or appear as amorphous, eosinophilic material. In the late stage, spleens remain grossly enlarged, and reticuoloendothelial cells predominate in the cell population of splenic tissue. There is variable fibrosis with residual necrotic and amorphous foci that may be surrounded by multinucleated giant cells.
Diagnosis
Drops in egg production and slight increases in mortality along with characteristic gross and microscopic lesions in the livers and spleens of dead or sick birds allow a strong presumptive diagnosis. Avian HEV is difficult to isolate. Electron microscopic examination of bile from affected birds may be useful. Virus particles, 30-50 nm in diameter, are seen in the bile of birds affected with HS syndrome. Finding virus particles in the bile substantiates the presumptive diagnosis.
PCR has been developed but is not commonly used to detect the virus in tissues. Developing a universal PCR primer should consider the genetic variation among avian HEVs. An ELISA test to detect antibodies to avian HEV has been developed but commercial ELISA kits are not available. Using polyclonal antisera and crude antigen preparations, agarose immunodiffusion (AGID) tests and immunofluorescence have been used to detect BLS agent, but the sensitivity and specificity of these tests are unknown. Antigen-capture ELISA using monoclonal antibodies to partially purified antigen prepared from the livers of chickens naturally infected with the BLS agent was found to be sensitive and specific for detecting BLS disease agent in faecal material, blood, and tissues.
Because subclinical infection with avian HEV is widespread in chickens, detection of antibodies in sera or virus antigen in faeces or tissues should be interpreted in conjunction with clinical signs and gross and microscopic lesions when implicating avian HEV as the cause of disease.
For liver lesions, fatty liver-haemorrhagic (FLH) syndrome is the main differential. In chickens with FLH syndrome, the liver is yellow, friable, and greasy on cut surface. Marked fatty degeneration of hepatocytes is seen microscopically. The main differentials for splenomegaly are lymphoid tumours (lymphoid leukosis, Marek’s disease) and bacterial septicemia, both of which have characteristic histological lesions.
Public health significance
There is no known public health risk from avian HEV. Infection of humans with avian hepatitis-E-virus has not been identified. Genomic sequences of avian HEV and HEVs from humans indicate that chickens are very unlikely to be a reservoir of human infections. In addition, rhesus monkeys were not susceptible to experimental infection with avian HEV.













