New rapid protection bird flu vaccine
An improved poultry influenza vaccine has been developed by researchers from the Pirbright Institute. Poultry World spoke to Professor Munir Iqbal, head of the Avian Influenza Virus group, to more fully understand this vaccine and its potential.
Many poultry flu vaccines protect birds from serious illness and death but do not prevent them from transmitting the virus. This vaccine, which is still under development, triggers a rapid immune response that protects chickens against signs of disease and reduces the amount of virus that they could pass on, which is a significant factor in halting the spread of bird flu. The vaccine would also be easier and less costly to produce than the traditional flu vaccines made in chicken eggs.
Novel technique vaccine
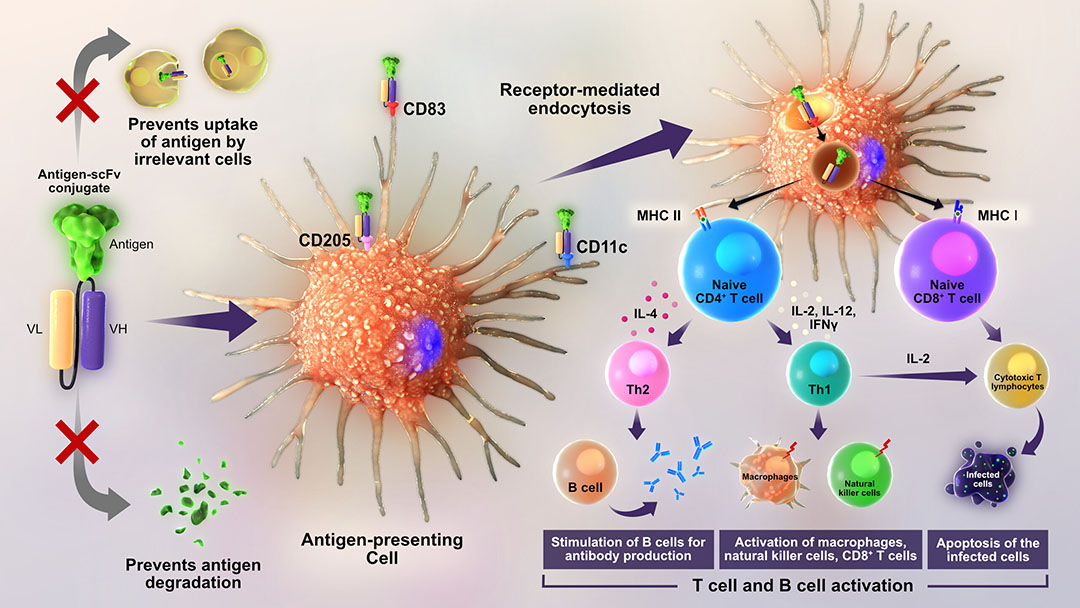
New methods have been developed to enhance the immune response that vaccines produce and reduce the amount of virus that birds shed into the environment. One technique involves tagging flu virus proteins with a marker that makes them easier for antigen-presenting cells (APCs) to capture. These immune cells can efficiently process the tagged proteins, resulting in a robust and long-lasting antiviral response in chickens.
Faster and stronger immune response
For the first time researchers at The Pirbright Institute, Surrey, UK, have shown that tagging the flu virus haemagglutinin (HA) protein and directing it to target a specific protein on the chicken APCs, called CD83, generates faster and stronger immune responses to the H9N2 bird flu strain compared to the current industry standard inactivated virus vaccine.
The results revealed that the vaccine was both fast-acting and effective. Birds produced antibody responses as early as 6 days after vaccination and they shed significantly less flu virus when challenged with a natural flu strain, indicating that the birds would be less likely to spread infection. High levels of protective antibodies were produced even when birds were given a reduced dose.
Using insect cells instead of chicken eggs
This vaccine will also be easier and less costly to manufacture. The tagged flu virus HA protein can be produced in a laboratory culture of insect cells rather than eggs to grow live vaccine viruses. “Therefore, no animals or derivatives from animals – the serum – will be required for the production of these next-generation vaccines,” says Iqbal, whose team is currently investigating the vaccine’s potential for commercial production and use in the field. “By targeting HA to chicken immune cells, we have generated a powerful addition to the armoury of poultry vaccines. Our improved vaccine could help prevent the spread of avian flu among vaccinated birds which is essential for protecting poultry welfare, increasing food production and reducing the risk of avian influenza spreading to humans,” said Iqbal.

Poultry World spoke to Professor Iqbal to ask some pertinent questions…
How many years have you and the team been working on this vaccine?
We have been working on developing different vaccine platforms to prevent avian influenza viruses for the last 17 years. Our work has included the development of herpesvirus of turkey and duck enteritis virus-based live bird flu vaccines which provide life-long immunity to either bird flu and Marek’s disease, or bird flu and duck enteritis. This recent and significant development is a targeted antigen delivery vaccine (TADV) whereby a flu protein (haemagglutinin) is targeted to the receptors on antigen-presenting cells (APCs) which produce an immune response. Such TADV has demonstrated stronger immune responses in chickens with good potential for commercialisation.
As the lead researcher, what has been the most exciting moment of progress or breakthrough in the development of this new vaccine?
The most exciting observation was that our targeted antigen delivery vaccine (TADV) – which contained only a recombinant protein (not whole virus) – induced faster, stronger and more long-lasting immunity in chickens compared to the available traditional inactivated avian influenza vaccines.
What is the key difference or highlight of this vaccine?
- Faster and stronger induction of immunity.
- Complete protection from clinical diseases.
- Significantly reduced shedding of virus, thus potentially reduced onward transmission, breaking the endemic cycle of the disease.
- Ability to produce broadly cross-reactive antibodies which could protect against multiple flu strains.
The key feature of the vaccine is that one dose of vaccine contains only a small amount (0.02 mg) of haemagglutinin (HA) protein of H9N2 influenza virus tagged with an antibody that specifically recognises chicken immune cells, known as antigen-presenting cells (APCs). Such targeting of the HA to chicken APCs enhances the capture and processing of the protein, preventing any unspecific uptake of the HA by non-relevant cells. This not only enhances the immune response but also provides the advantage of ‘dose sparing’, meaning that a smaller amount of vaccine is required to induce stronger and long-lasting antigen-specific immunity, which is more cost-effective.
Tell us more about the vaccine production process and why it is more cost-effective?
The tagged HA used in the vaccine is produced in an insect cell culture system which can be propagated at room temperature (22-28°C) in the bioreactors or even in a shaking flasks incubator and does not require carbon dioxide or other expensive additives like those required for mammalian cell culture systems. Unlike the traditional inactivated virus vaccine, this vaccine does not use embryonated eggs or high containment facilities which further reduces the manufacturing cost.
In practice, what other benefits will poultry producers gain from using this vaccine?
There are numerous benefits, which include:
What are the implications in the field?
This vaccine will provide robust immunity in vaccinated flocks against specific pathogens, such as avian influenza. This will reduce economic losses, as well as improve the health and welfare of the birds. The vaccinated birds will also shed significantly smaller amounts of virus when challenged with infectious virus. This reduces virus load in the environment and will result in lower disease incidence in birds, leading to a reduced risk of zoonotic transmission from infected birds to humans.
Could this new vaccine lower the threshold for use in countries that are now reluctant to use it (e.g. the EU)?
The use of traditional inactivated virus vaccines is prohibited in some countries because these vaccines do not allow differentiation between vaccinated and infected birds (DIVA). Such DIVA incompatibility of the vaccine can interfere with the disease surveillance system of the country. The new vaccine (TADV) contains only one viral protein (haemagglutinin) and is compatible with serological tests detecting antibodies against other viral proteins. Thus, this vaccine is compatible with the DIVA strategy which is one of the important requirements for obtaining the regulatory approval necessary for the licensing of a poultry avian influenza vaccine in some countries.
What influenza types are targeted and can the vaccine be adapted to protect against other/new variants?
Yes, as a first step we have produced a prototype targeted vaccine for bird flu (H9N2 virus) which induces strong immunity against the H9N2 virus variants. Our future work will involve producing TADV against a range of different bird flu subtype/serotypes, as well as other poultry pathogens, like the Newcastle disease virus, infectious bursal disease virus and infectious bronchitis virus.
When will the vaccine reach the market/approval?
’We are working jointly with a commercial vaccine producer to take the vaccine from the laboratory into the field. The experimental work and documents required for vaccine regulatory approval are in progress and we anticipate that the vaccine will be available on the market soon.












