Setting a proper hatchery vaccination because every chick counts

A statistical analysis of relevant key hatchery indicators related to the vaccination process showed that proper vaccine preparation conditions have a positive impact on a successful hatchery vaccination process.
Today, vaccinations are concentrated at hatchery level to avoid the disadvantages of traditional farm-vaccination. This presents hatcheries with increasing challenges because they need to integrate new processes into their production while maintaining their goals of quality and productivity. On top of this, given current staffing shortages and the time needed for new employees to learn the procedures, the quality of the hatchery vaccination process can be compromised.
According to our data, about a third of the hatchery operators trained in one year were new appointments. Therefore, the impact of untrained staff failing to attend to any detail during the vaccination process can be significant.
The uniformity of the birds’ immune response to disease depends on a consistently high standard of hatchery vaccination. Bearing this in mind, hatchery staff mainly focus on correct equipment operation and the quality of the vaccine injection or spray. However, the process starts in the vaccine preparation room. Indeed, its condition in terms of cleanliness and organisation will in turn always impact the preparation procedures for a vaccine solution that, ultimately, will be administered to the birds. We used our global database from the C.H.I.C.K. Program service to investigate this correlation.
The C.H.I.C.K Program is the best-in-class service provided exclusively by hatchery specialist Ceva around the world to its customers’ partners. For this purpose, relevant key hatchery indicators related to the vaccination process were evaluated when a frozen Marek’s vaccine administered by subcutaneous injection was used. These key hatchery indicators were statistically analysed.
Vaccine preparation-room conditions
To ensure a clean and organised vaccine preparation room the starting point for the control is to aim to have 100% of birds properly vaccinated on a consistent basis. For example, if the vaccine room is unhygienic, not ventilated and without positive air pressure (to avoid cross-contamination due to proximity to the chick processing area), and if it is also used as storage room for other materials unrelated to the vaccination process, this immediately sets off a chain reaction along the entire vaccination process.
An inadequate vaccine preparation room negatively affects proper vaccine preparation and, as a consequence, the vaccine administration. As a result, the birds may be correctly injected but with a non-viable vaccine solution and therefore flock immunisation will be compromised.
Following procedures for vaccine reconstitution
All vaccine manufacturers are expected to provide straightforward procedures for vaccine reconstitution. However, for hatchery operators, it is not enough just to have the right procedure. The rapid turnover of personnel working in hatcheries makes it necessary to provide continuous personal training with regular follow-ups. According to our data, about a third of the hatchery operators trained by our national teams in one year were new appointments. Therefore, having set regular training sessions and providing training materials will help to ensure that the procedures are understood and consistently applied by the hatchery staff.
Data set and statistical analysis
We conducted an analysis of C.H.I.C.K. Program hatchery vaccination scores related to vaccine room conditions, together with the scores obtained for the process of reconstituting the vaccine. The study period was from January 2021 to September 2022, so 21 months of audits conducted in a large number of hatcheries from four different regions: Western Europe (W. Europe), Latin America (Latam), Asia (Asia), Eastern Europe, Africa and the Middle East (Eemea). The dataset comprised about 7,454 hatchery vaccination audits in 65 countries. The two key variables studied were ‘vaccine preparation room conditions’ and ‘vaccine preparation procedures for frozen vaccines’. The results per region are shown in Figure 1.
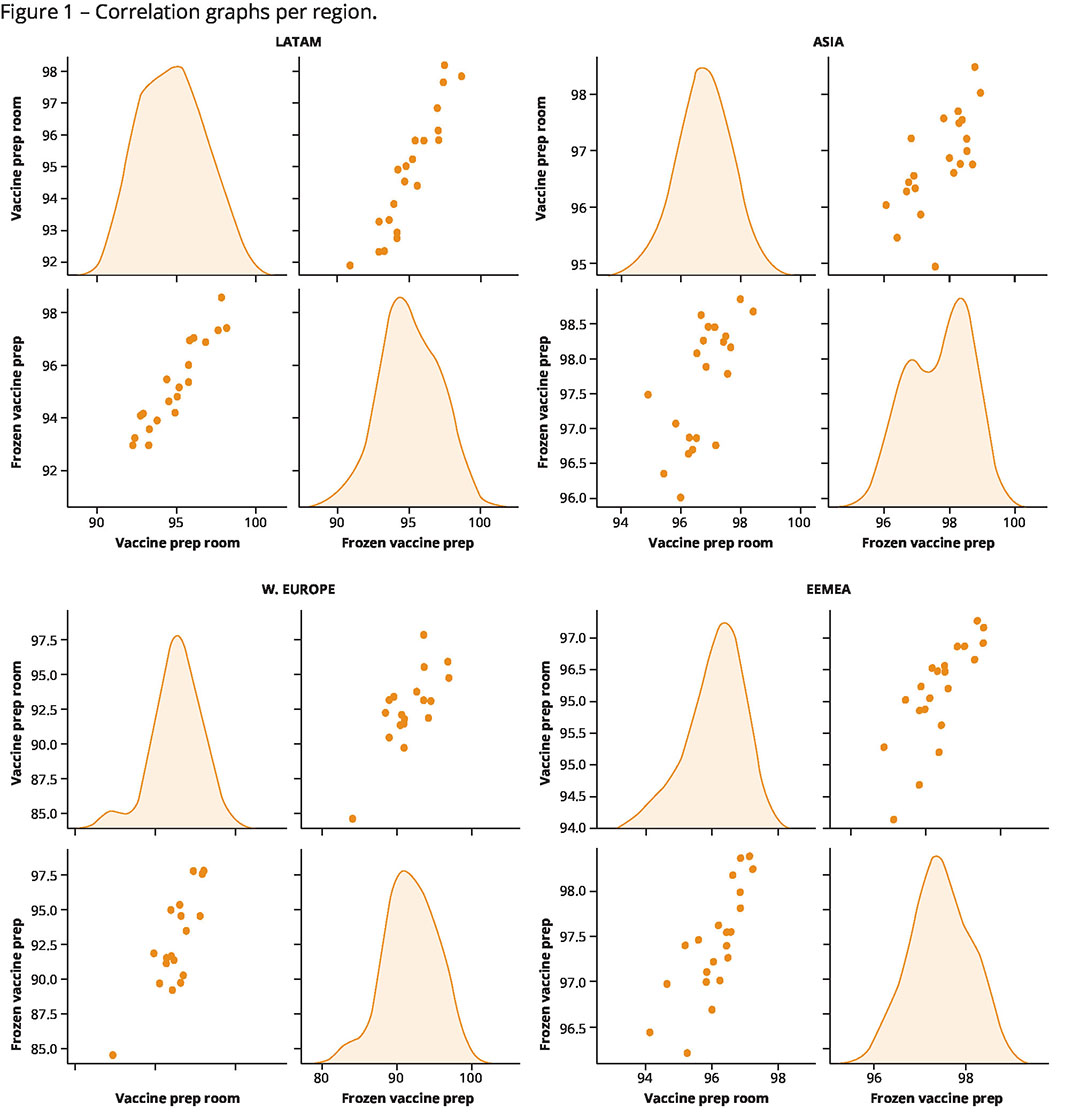
The statistics were obtained using Python statistical software. According to the dataset, a significant (p<0.05) medium-strong and positive correlation was found between the ‘vaccine room conditions scores’ and the ‘frozen vaccine preparation scores’ for all four zones (Latam, Asia, Europe and Eemea). With the strongest positive correlation seen in Latin America (Pearson’ correlation coefficient = 0.93), followed by Europe and Eemea (Pearson’s correlation coefficient = 0.78 and 0.79, respectively) and finally, Asia (Pearson coefficient = 0.67).
Positive impact
The success of the hatchery vaccination process starts in the vaccine preparation room. From the results it may be concluded that there is a strong correlation (p>0.7) between the scores for vaccine preparation room conditions and vaccine reconstitution procedures which is clearly explained by the human factor (operation and error).
According to our analysis, a properly reconstituted vaccine solution will more often come from a good vaccine preparation-room. In other words, when care is taken in the workplace, the same will apply with respect to the reconstitution procedures. Therefore, appropriate vaccine preparation conditions have a positive impact, resulting in a successful hatchery vaccination process. This makes it imperative that hatchery staff receive adequate training and continuous support to ensure that all birds are properly vaccinated. Because every chick counts.






