Video 7: Avilamycin’s global footprint, quality and particle size
In this episode, we sit down with George Tice, executive director public policy Elanco to learn about Avilamycin’s global footprint, quality and particle size.
Avilamycin was introduced across the world in 1987 and is currently being used in more than 40 countries, including the U.S., Brazil, Canada, and Japan, and therefore in billions of broilers every year.
What about its safety? With the long history of use of Avilamycin, there have been no concerns regarding antimicrobial resistance in humans. And that’s because it belongs to the antimicrobial class Orthosomycins. And, that class is not used in human medicine. So, no antimicrobial resistance concerns. Secondly, no residue concerns. It has an excellent residue profile and that’s because there is very little absorption in the bird’s gastrointestinal tract.
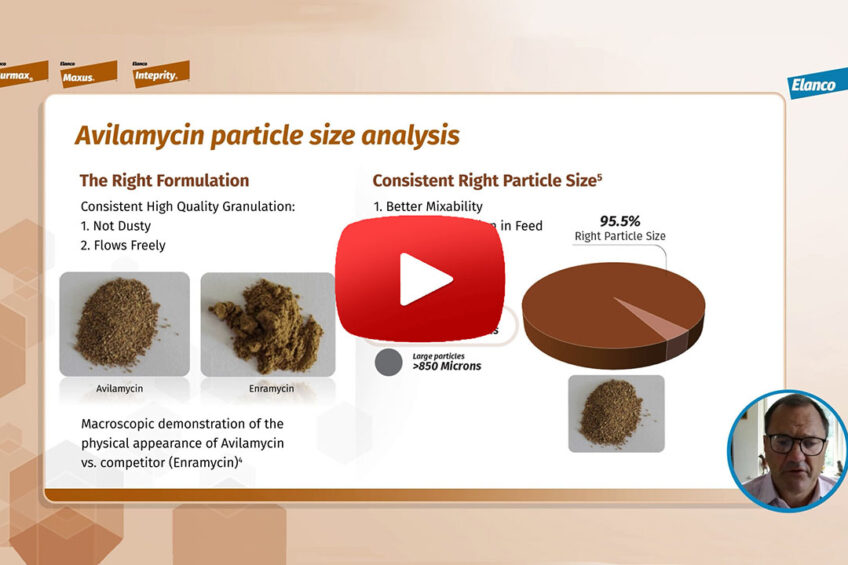
What about its quality? Avilamycin is manufactured in Speke, England, and it’s a complex manufacturing process that the plant in Speke has mastered. And we ensure high quality and product consistency. What we mean by that, firstly, is the right potency in every batch with the right amount of active ingredient. Secondly, the right formulation. The components of the right formulation include a high-quality granule, which is not dusty and flows freely, and the right particle size distribution, leading to better mixability and better distribution in feed.
What about its traceability? Elanco can trace the identity, history and source of all the raw materials, ingredients and feed stocks used in the manufacturing of Avilamycin. And of course, we keep meticulous records at all stages in the production system. So, Avilamycin has a long history of safety, consistent potency, granular and particle size formulation and traceability. This provides customers with peace of mind by delivering the correct dose to every bird, every day during the treatment period.
Take a look at the previous 3 videos from the series
- Video 4: Efficacy of long term use of Avilamycin in broiler production
- Video 5: Why Avilamycin poses no risk to human antimicrobial resistance
- Video 6: Why Avilamycin is the responsible choice; WHO guidelines