Balanced intestinal microflora help prevent necrotic enteritis
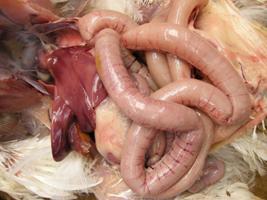
Necrotic enteritis is a disease affecting chickens and turkeys throughout the world. Certain factors are known to predispose birds to this disease. Keeping a healthy balance of intestinal microflora seems to be a key element in the prevention strategy. Affected flocks need to be treated to minimise death losses.
By Dr. Tahseen Aziz, Rollins Animal Disease Diagnostic Laboratory, Raleigh, NC, USA and Dr. H. John Barnes, College of Veterinary Medicine, NC State University, Raleigh, NC, USA
Necrotic enteritis (NE) is an enteric bacterial disease of chickens, turkeys, and a few other avian species caused by Clostridium perfringens. The disease is characterised by damage to the intestinal mucosa by toxins produced by the causative bacteria. It is worldwide in distribution and causes considerable financial losses to broiler producers due to mortality and, in its milder subclinical form, poor growth and feed utilisation. In commercially raised broiler chickens, clinical disease usually occurs between 2 and 5 weeks of age.
The causative bacterium
Necrotic enteritis is caused by C. perfringens, a gram-positive, rod-shaped, spore-forming, anaerobic bacterium. C. perfringens is ubiquitous, found in used litter, soil, and intestinal tracts of healthy birds. Feed and litter contaminated with large numbers of C. perfringens have been convincingly implicated as a source of infection. The disease occurs when C. perfringens overgrows in the intestinal tract and produces potent toxins that severely damage the intestinal mucosa. Toxins absorbed from the intestinal tract produce a toxemia (toxin in blood), which is responsible for death of the bird. Thus, NE is a type of “enterotoxemia”.
Necrotic enteritis is caused by C. perfringens, a gram-positive, rod-shaped, spore-forming, anaerobic bacterium. C. perfringens is ubiquitous, found in used litter, soil, and intestinal tracts of healthy birds. Feed and litter contaminated with large numbers of C. perfringens have been convincingly implicated as a source of infection. The disease occurs when C. perfringens overgrows in the intestinal tract and produces potent toxins that severely damage the intestinal mucosa. Toxins absorbed from the intestinal tract produce a toxemia (toxin in blood), which is responsible for death of the bird. Thus, NE is a type of “enterotoxemia”.
C. perfringens is divided into five toxinotypes (A, B, C, D, and E) based on four major toxins (alpha, beta, epsilon, and iota). The majority of isolates from NE cases are type A, with a few cases caused by type C. Alpha toxin produced by types A and C, beta toxin produced by type C, and possibly other toxins produced by the organism are responsible for the damage to the intestine, enterotoxemia, and death of the bird.
Alpha toxin, produced by C. perfringens toxinotype type A, is an important virulence factor in the pathogenicity of the organism. It is a phospholipase that hydrolyses phospholipids in membranes of red blood cells, white blood cells, platelets (thrombocytes), endothelial cells, and muscle cells; because of its hydrolytic property, the toxin is hemolytic, cytotoxic, necrotising, and potentially lethal. Large variations in the amount of alpha toxin are produced by different isolates of C. perfringens in vitro, but isolates of the same genetic type produce the same amount of alpha toxin.
However, the amount of alpha toxin produced by C. perfringens isolated from NE lesions is not significantly different from the amount of the toxin produced by isolates from the intestine of healthy birds. Recently, another novel toxin (NetB) has been identified in certain strains of C. perfringens. Initially, this toxin was thought to be the major and critical virulence factor in C. perfringens strains capable of causing NE, but recently published research indicates that NetB-negative strains are also capable of causing NE in experimentally challenged chickens. Certainly, the role of NetB as a virulence factor in the pathogenesis of NE needs further investigation.
Predisposing factors
C. perfringens is found in the small intestine of healthy chickens, usually in small numbers. It colonises the intestines of broiler chickens within a few hours after hatching and the numbers of the organism increase gradually after initial colonisation. Events leading to excessive growth (multiplication) of C. perfringens in the small intestine, with subsequent production of toxin and damage to the intestinal mucosa, are poorly understood.
However, certain factors are known to predispose birds to NE. It is presumed that these factors promote excessive growth of C. perfringens through mechanisms that are yet to be clarified. These factors include:
1. Small intestinal coccidiosis. Protein-rich exudate leaking from the damaged mucosa may provide necessary nutrients for the growth of C. perfringens. The minimum growth requirement of C. perfringens includes several amino acids and many growth factors and vitamins. In modern, intensive poultry production, coccidiosis is probably the most important predisposing factor under field conditions. In our experience, NE cases are commonly associated with varying degrees of Eimeria maxima infection.
2. Diets high in cereal grains such as rye, wheat, and barley contain high levels of indigestible, water soluble, non-starch polysaccharides. These grains are believed to predispose to NE by increasing the viscosity of the intestinal contents.
3. High amounts of animal protein, such as fish meal and bone meal, in the diet increase the risk of NE compared to feed formulated with plant sources of protein. The increased risk associated with animal sources of protein has been attributed to high glycine and methionine levels in animal protein; both of which enhance C. perfringens growth in vitro
4. Animal fat (mixture of lard and tallow) increases the numbers of C. perfringens in the ileum, compared with soy oil.
5. In turkeys, NE has been associated with ascaridiasis, coccidiosis, and hemorrhagic enteritis.
6. Management factors that include high stocking density, high fibre litter, and sudden changes in diet also increase the incidence of NE.
However, in many NE outbreaks none of these factors can be implicated as predisposing the broiler flock to the disease. Even when known predisposing factors are considered, it is still difficult to reproduce the disease experimentally. There are probably other predisposing factors that are yet to be identified.
Clinical signs and lesions
Typically NE has a short clinical course. The history usually states that birds in the flock are found dead without premonitory clinical signs. Some birds may appear listless and lethargic for a few hours before death. Birds affected with the mildest, subclinical form of NE, may not die but show reduced weight gains and higher feed conversion ratios, with increased condemnations at the processing due to liver lesions.
Typically NE has a short clinical course. The history usually states that birds in the flock are found dead without premonitory clinical signs. Some birds may appear listless and lethargic for a few hours before death. Birds affected with the mildest, subclinical form of NE, may not die but show reduced weight gains and higher feed conversion ratios, with increased condemnations at the processing due to liver lesions.
The lesion in the intestinal tract is usually confined to the jejunum and ileum. It varies in appearance from bird to bird depending on the severity of infection, stage of development, presence or absence of coccidiosis, and freshness of the carcass. When birds have NE it is best to examine euthanised or fresh dead birds for lesions. Once the intestine starts to decompose after death, NE lesions tend to be less obvious. The jejunum and ileum may appear dilated, have a thin, friable wall, and be filled with gas or contain green or red-tinged fluid admixed with debris. In mild cases the intestinal mucosa has a granular or somewhat roughened appearance.
In severe cases the mucosa is discoloured green, brown, or red–brown, and it appears markedly thickened, roughened, or velvety. A green, red, brown, or pink pseudomembrane may cover and loosely adhere to the mucosa; pieces of the membrane may slough into the lumen. In rare cases, spots (multifocal necrosis) in the liver and an enlarged gall bladder with white spots in the wall (cholecystitis) may be seen.Microscopically, the hallmark lesion of NE is diffuse necrosis of the mucosal villi in the affected segment of the small intestine. Usually all of the villi are necrotic and replaced by eosinophilic (pink) necrotic debris, with numerous large, rod-shaped bacteria along the outer margin of the debris. In severe cases a fibrinonecrotic membrane adheres to the underlying viable mucosa. In cases of concurrent coccidiosis, developmental stages of Eimeria spp. are seen within the necrotic debris and lamina propria. If there are gross lesions in the liver or gall bladder, necrosis of hepatic tissue and the wall of the gall bladder with numerous large, intralesional, rod-shaped bacteria is seen microscopically.
Diagnosing NE
Gross and microscopic lesions are characteristic and diagnostic of NE. Grossly, lesions of NE can resemble those caused by Eimeria brunetti; however NE can be confirmed histologically. Remember that NE and coccidiosis often occur concurrently. Isolation of C. perfringens may be attempted by ligating an affected segment of intestine at both ends and delivering it as soon as possible to a laboratory for anaerobic bacterial culture. Do not freeze the specimen, as the vegetative cells of C. perfringens die at freezing temperature. Alternatively, a swab in an anaerobic transport medium may be used to culture the mucosal lesions. The swab should be delivered to the diagnostic lab as soon as possible. The results of bacterial culture need to be interpreted in the context of clinical history, gross lesions, and preferably microscopic lesions.
Gross and microscopic lesions are characteristic and diagnostic of NE. Grossly, lesions of NE can resemble those caused by Eimeria brunetti; however NE can be confirmed histologically. Remember that NE and coccidiosis often occur concurrently. Isolation of C. perfringens may be attempted by ligating an affected segment of intestine at both ends and delivering it as soon as possible to a laboratory for anaerobic bacterial culture. Do not freeze the specimen, as the vegetative cells of C. perfringens die at freezing temperature. Alternatively, a swab in an anaerobic transport medium may be used to culture the mucosal lesions. The swab should be delivered to the diagnostic lab as soon as possible. The results of bacterial culture need to be interpreted in the context of clinical history, gross lesions, and preferably microscopic lesions.
Effective treatment
Flocks affected with NE are usually treated with antibiotics administered via drinking water. If C. perfringens is sensitive to the antibiotic, the flock should rapidly respond to treatment. The dose and duration of treatment recommended on the package of the medication should be followed to ensure efficacy of the treatment. Penicillin, tetracycline, lincomycin, and erythromycin are drugs of choice for treating NE.
Flocks affected with NE are usually treated with antibiotics administered via drinking water. If C. perfringens is sensitive to the antibiotic, the flock should rapidly respond to treatment. The dose and duration of treatment recommended on the package of the medication should be followed to ensure efficacy of the treatment. Penicillin, tetracycline, lincomycin, and erythromycin are drugs of choice for treating NE.
| Microscopic lesion in the small intestine of a 17-day-old broiler chicken affected with necrotic enteritis that has concurrent coccidiosis caused by Eimeria maxima. Villi are totally destroyed (necrotic), with numerous bacteria (blue material) in the necrotic debris. Several developmental stages of Eimeria can be seen in the remaining mucosa and necrotic debris. | Microscopic lesion in the small intestine of a 31-day-old broiler chicken affected with necrotic enteritis. There is diffuse necrosis of villi, which are destroyed and replaced by pink necrotic debris. The blue staining on the surface of the necrotic debris is the causative organism (Clostridium perfringens). |
If the flock does not respond to treatment within 24-48 hours, the following possibilities need to be considered: (1) incorrect diagnosis of NE, (2) resistance of C. perfringens to the antibiotic, (3) incorrect dose of medication, and (4) presence of concurrent disease, such as coccidiosis. C. perfringens is sensitive to several in-feed antibiotic growth promoters. Use of these antibiotics as feed additives suppresses the numbers of C. perfringens in the intestinal tract and reduces the incidence of NE.
However, since 1997 the European Union has banned the use of several growth promoting antibiotics including avoparcin, ardamycin, bacitracin, virginiamycin, tylosin, and spiramycin, which has been associated with increased occurrence of the NE. C. perfringens is sensitive to the ionophorous coccidiostats monensin, salinomycin, and narasin. Incorporating these coccidiostats into the feed is effective in reducing the number of C. perfringens in the intestine and protecting against experimental NE.
Prevention strategy
The intestinal tract of birds contains several bacteria that compete with each other in the intestinal environment; NE occurs when C. perfringens overgrows in the intestinal tract. Keeping a healthy balance of intestinal microflora seems to be a key element in NE prevention. Giving certain beneficial bacteria (particularly Lactobacillus spp.) to birds is effective in preventing, or at least reducing, the severity of NE. So-called “competitive exclusion” products containing different bacteria are available commercially and are worth trying on farms with recurring NE problems. Competitive exclusion products should be given to the birds as soon as possible after hatching. Beneficial bacteria in these products may reduce colonisation of C. perfringens in young chicks.
The intestinal tract of birds contains several bacteria that compete with each other in the intestinal environment; NE occurs when C. perfringens overgrows in the intestinal tract. Keeping a healthy balance of intestinal microflora seems to be a key element in NE prevention. Giving certain beneficial bacteria (particularly Lactobacillus spp.) to birds is effective in preventing, or at least reducing, the severity of NE. So-called “competitive exclusion” products containing different bacteria are available commercially and are worth trying on farms with recurring NE problems. Competitive exclusion products should be given to the birds as soon as possible after hatching. Beneficial bacteria in these products may reduce colonisation of C. perfringens in young chicks.
Preventing coccidiosis, whether by coccidiostats or vaccination, is probably the most important measure that should be taken to prevent NE. When a coccidial vaccine is administered, it is extremely important to follow the instructions of the vaccine manufacturer to avoid excessive exposure of the birds to Eimeria oocysts. Particular attention needs to be paid to the vaccination dose, maintenance and calibration of vaccinating equipment, and management of the flock during the first two weeks post-vaccination. We have encountered cases of concurrent NE and coccidiosis in 2-3 week old chickens in which a coccidial vaccine had been administered in the hatchery. Prevention of NE involves an intervention strategy that takes into consideration all of the factors that may increase the risk of the disease.













