Campylobacter trial begins
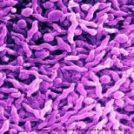
Following Investigational New Drug (IND) approval by the US FDA, Ace Biosciences and US Naval Medical Research Centre begin a Campylobacter trial.
The US Naval Medical Research Center and the infectious diseases company, ACE BioSciences, have announced that their first subjects are being dosed in a trial to establish a vaccine challenge model for the evaluation of Campylobacter vaccines. This includes ACE BioSciences’ leading product, the Travellers’ Diarrhoea (TD) vaccine ACE393.
ACE393 is on track to become the world’s first commercial vaccine for Travellers’ Diarrhoea caused by Campylobacter infection. The aim of the challenge trial is to establish the appropriate dose of bacteria needed to produce a response that is representative of the natural disease state, whilst posing minimal risk to volunteers.
It is anticipated that the trial will be completed in the second half of 2007.
Related links:
Related articles:













