Part 1: Bursa of Fabricius is a visual indicator
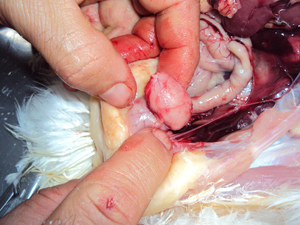
The bursa of Fabricius is a lymphoid organ which plays an important role in developing immunity against mainly Gumboro in chickens. But does its size say anything? And can we easily correlate its changes to biological conditions? In this first of a series of two articles, the question is answered if differentiation in vaccination can be based on its appearance.
By Dr Christophe Cazaban and Dr Yannick Gardin, CEVA Sante Animale, France
The cloacal bursa was given its name to pay tribute to Dr Hieronymus Fabricius, an Italian anatomist who used to work in Padua in the late 16th, early 17th century. He was the first to notice this small organ on the top of the cloaca in birds, but he did not know its actual role. The importance of the bursa of Fabricius (BF) as primary lymphoid organ has meanwhile been well described. Specifically in young birds it plays an important role in developing immunity.
Development of the BF
Basically, the BF is going through three consecutive development stages (see Figure 1):
Basically, the BF is going through three consecutive development stages (see Figure 1):
• A growth phase during the first three weeks of age;
• A “plateau” phase between the 4th and the 8th weeks of age;
• A slow, but steady regression phase from the 9th week of age onwards, until nearly disappearing when the sexual maturity is in place.
Following colonisation of the BF by an infectious bursal disease virus or IBDV (Gumboro virus) changes will be observed in a sequential way. In the acute phase within four days post-infection (PI), the BF size is multiplied by 2 to 3 times: swelling of the plicae, sometimes with some gelatinous material inside. On the 5th day PI, the BF is usually already back to its initial size.
Between the 8th and the 10th day PI, the BF gets atrophied by 3 to 6 times. The recovery phase can last up to 35 days PI. The actual duration of the recovery process mainly depends upon the virulence of the virus strain, on the quantity of virus invading the BF, and on the age at colonisation. In other words, an attenuated strain of IBDV (e.g. a vaccine strain) will colonise the bursa as well. However, the recovery process will be much faster (approx. seven days).
BF size
Before discussing enlargement (or atrophy), and to compare figures, one should know what is the physiological size of the BF. Another point to clarify is the influence of external parameters, like age, breed, sex, rearing conditions on the BF size. Published documents describing the normal size of the BF using actual figures, are hardly available. At least, Glick in 1956 extensively studied the BF in several chicken breeds over time. By keeping meat-type and laying-type pedigree birds under field conditions, the author recorded the BB ratio (bursa weight to body weight, expressed in %).
Before discussing enlargement (or atrophy), and to compare figures, one should know what is the physiological size of the BF. Another point to clarify is the influence of external parameters, like age, breed, sex, rearing conditions on the BF size. Published documents describing the normal size of the BF using actual figures, are hardly available. At least, Glick in 1956 extensively studied the BF in several chicken breeds over time. By keeping meat-type and laying-type pedigree birds under field conditions, the author recorded the BB ratio (bursa weight to body weight, expressed in %).
As expected, the BB ratio is increasing in the first weeks in meat-type breeds, due to a strong bursa development, and a relatively slow body development. From the 6th week of age onwards, the BB ratio goes down, due to a stabilisation of the BF development, while the body growth is strong. A few differences in the average BB ratio can be noticed between males and females: most of the time, the BB ratio in males is lower than in females: this is probably linked to the heavier body weight in males. Later samplings showed a high individual variability in bursa weight for a given age.
In laying-type birds, Glick was able to identify an influence of the breed on the BB ratio. For instance, in a 6-week long study, the BB ratio was constantly greater in White Leghorn than in Rhode Island Red female chickens.
In addition, when keeping the same breed in cages instead of litter, the BB ratio figures did vary. The average BB ratio kept steady between 4 and 6 weeks of age when reared on litter, whereas it dropped quickly when reared in cages.
Major genetic changes
Today, this is a 50-year old database, and the genetic improvements have dramatically modified the shape and the growth potential of the body in chickens. For instance, the weight target in meat type birds was 467 g in the Glick study, and it is more than twice this figure nowadays. In heavy broilers, one rearing day was saved yearly in the last 30 years. These parameters do certainly impact on the BB ratio figures recorded today, compared to previously.
Today, this is a 50-year old database, and the genetic improvements have dramatically modified the shape and the growth potential of the body in chickens. For instance, the weight target in meat type birds was 467 g in the Glick study, and it is more than twice this figure nowadays. In heavy broilers, one rearing day was saved yearly in the last 30 years. These parameters do certainly impact on the BB ratio figures recorded today, compared to previously.
In an experiment by Heckert, the impact of the rearing density of broiler chickens over the BB ratio was recorded at 42 days. Avian x Avian day-old chicks, kept under experimental conditions as close to field conditions as possible, were allocated to pens with varying birds density: 10, 15, and 20 birds per m². Feeding and drinking spaces were designed accordingly. They tested three replica of three groups to get a better statistical relevance of the experiment. They demonstrated that because of stress, the higher the density, the lighter the BF. The size of the BF is also reduced by a too cold, or a too hot environment.
Monitoring health status
According to researcher Bennett, bursometry is a poorly accurate technique compared to BB ratio measurement. In addition, Moraes stated that there is no direct link between the bursa diameter and any disease or any vaccination. The bursa diameter does not correlate with histopathology lesion scores either. These authors even considered that the bursometry was inadequate to properly assess any vaccine pathogenicity.
According to researcher Bennett, bursometry is a poorly accurate technique compared to BB ratio measurement. In addition, Moraes stated that there is no direct link between the bursa diameter and any disease or any vaccination. The bursa diameter does not correlate with histopathology lesion scores either. These authors even considered that the bursometry was inadequate to properly assess any vaccine pathogenicity.
The aim therefore would be to get a standard of a “good”, or “acceptable”, or “physiological” BB ratio in order to be able to assess the health status of a chicken flock in field conditions (see Table 1).
It is obvious from these figures that it is quite difficult to define a procedure for monitoring the health status of a flock in field conditions based on BB ratio recording. There is neither a consensus on the figure of a healthy BB ratio itself, nor on the method for recording it. Some authors even considered that the BB ratio is more valid in experimental conditions than in the field. When comparing a treated (or vaccinated) group to controls: bursa in a treated group would be considered atrophied if the mean BF weight is two standard deviations below the mean of the controls. Even in experimental conditions, there is no consensus on the proper timing to assess the BB ratio after contact with IBDV. Various researchers advice differently in a range from 7 to 20 days.
No standardised protocol
To conclude, there are many roadblocks to set up universal BF size standards. There is a lot of studies assessing the bursa atrophy (eg, through a drop in BB ratio figures) following a vaccination using a live attenuated IBD vaccine. However, to actually draw conclusions, there is neither a standard for BB ratio per breed, per week. Its use under field conditions is therefore questionable. Also there’s not a standardised protocol (timing post exposure) in laboratory conditions.
To conclude, there are many roadblocks to set up universal BF size standards. There is a lot of studies assessing the bursa atrophy (eg, through a drop in BB ratio figures) following a vaccination using a live attenuated IBD vaccine. However, to actually draw conclusions, there is neither a standard for BB ratio per breed, per week. Its use under field conditions is therefore questionable. Also there’s not a standardised protocol (timing post exposure) in laboratory conditions.
Any figures recorded in experimental conditions can hardly be compared with other studies. As a consequence, it can be concluded that it is not possible to clearly classify the virulence of the IBD vaccines using the BB ratio. BF weight, BB ratio, body weight and antibody titer evaluated before or after challenge with IBDV, are not enough to consistently and conclusively differentiate or estimate the protection given by vaccination.
* References are available upon request to the authors













