Mycotoxins, what makes them different?
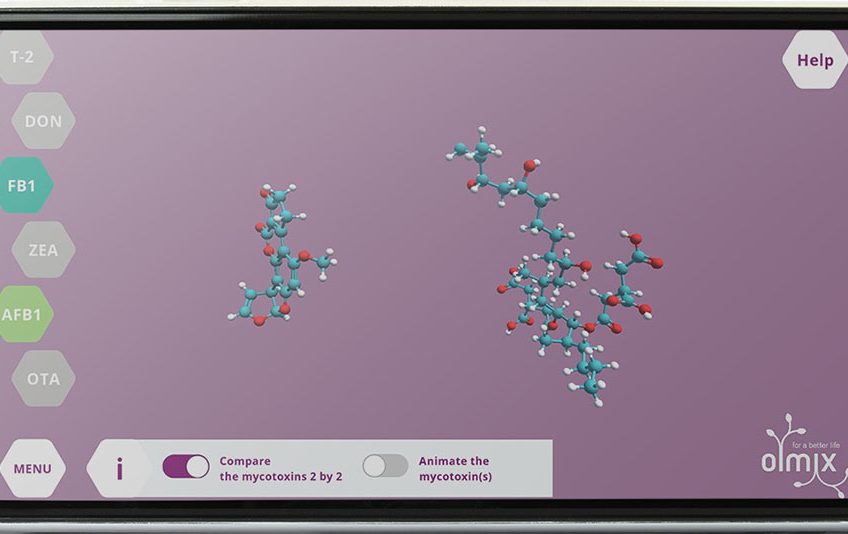
The differences of composition and conformation (3D-shape due to natural rotation) of mycotoxins, explain the differences in terms of size, volume and mobility. This will have a great impact on their intestinal absorption in terms of toxicity and adsorbent affinities.
Mycotoxins are toxic compounds synthetized by fungi, either mainly at pre-harvest by Fusarium, or mainly at post-harvest by Aspergillum and Penicillium. Feed materials can be contaminated by different mycotoxins, depending on the cultivation method and climate conditions. Looking at the structure of mycotoxins, it is obvious they have totally different physiochemical characteristics.
Mycotoxin physicochemical characteristics
Aflatoxins are known for being the smallest mycotoxin and essentially planar, which significantly facilitate their intestinal absorption and interaction with adsorbents. Ochratoxins are also planar but with a protuberant part that makes them 1.5 times bigger than aflatoxins. Moreover, the amide group present in ochratoxins provides flexibility to this type of mycotoxins and depending on the environment (energy available) ochratoxin A can adopt four different conformations.
Zearalenone can also be qualified as flexible mycotoxin as it can adopt two conformations or different organisations of the protuberant part, located around the ketone group of C7 carbon.
Fumonisins are the biggest mycotoxins, grape shaped with a very long and thin chain (like fatty acids) and three branched chains at one end. Fumonisin B1 can adopt two different conformations depending on the presence of lipids or not, which will have a major impact on the possible interactions in the animal body. Trichothecenes are globular mycotoxins due to the presence of an epoxy group. T-2 toxin (a type-A trichothecene) is voluminous as it is composed of two side chains branched on the globular structure. Deoxynivalenol (type-B trichothecene) is composed of the globular structure without side chains which makes it very rigid and complexify the possibilities of interactions.
Intestinal absorption of mycotoxins
Mycotoxins will have different behaviour inside the gut depending on the environmental conditions and their own physicochemical specificities. According to a dynamic model simulating the pig intestinal tract, most of mycotoxin absorption happens in the upper part of the intestine, meaning in the jejunum (70-85%) rather than the ileum (15-30%). The intestinal absorption mechanism of mycotoxins mainly uses the transcellular transport way or paracellular transfer and rarely the endocytose.
Difference in pH gradient
The physico-chemical properties (mainly volume and polarity/hydrophobicity) of mycotoxins explain the different transport ways used and the rate of intestinal absorption between mycotoxins. Studies on monogastrics report that aflatoxin B1, deoxynivalenol, and ochratoxin A are rapidly and passively absorbed at high rates, respectively >80%, >55% and >65% of the level analysed in the feed, while fumonisin B1 absorption is active, dependent to cholesterol or bile salts transports, and limited (< 6% of feed level). Thus, a significant portion of non-absorbed mycotoxins remains within the lumen, exposing the gut epithelium to various alterations. The difference in pH gradient in the gut between swine and poultry also affect the fate of mycotoxins in the gut, for instance deoxynivalenol intestinal absorption is very limited in poultry (5-20%) compared to swine (>55%).
The exact interaction between adsorbing agents and mycotoxins is well described only for a limited number of situations
Different adsorbent-mycotoxin affinities
Mycotoxin-adsorbing agents are large molecular weight compounds that are able to bind mycotoxins without dissociating along the gastrointestinal tract of the animal, so that the toxin-adsorbing agent complex is eliminated via the faeces. Many mineral and/or organic adsorbing agents are available in the market. Their mode of action is based on intermolecular interactions (toxin-binder) that depend on electrostatic/hydrophobic interactions (hydrogen or ionic binding and Van der Waals forces) linked to mycotoxin composition and conformation.
Limited number of situations
The exact interaction between adsorbing agents and mycotoxins is well described only for a limited number of situations. Organic adsorbing agents such as yeast cell walls are frequent in the feed market for their ability to complex with zearalenones and ochratoxins without reducing the bioavailability of nutrients. They are mostly composed of polysaccharides (beta-glucans and mannan-oligosaccharides (MOS)) involved in the formation of both hydrogen bonds and van der Waals interactions with mycotoxins. The interaction between the yeast cell wall and zearalenone seems to involve hydrogen bonds and Van der Waals force between β-glucan and zearalenone, though no direct correlation between yeast composition (β-glucan and MOS content) and adsorption capacity has been found. The exact mode of binding between yeast cell wall and zearalenone is controversial whereas it remains unclear for ochratoxins.
Phyllosilicates
Phyllosilicates (smectites) minerals are the most frequent mycotoxin adsorbing agent in the market. The interlayer space (d-spacing), between the alumino-sillicates layers that compose smectites, allows the entrance and the efficient binding of planar molecules like aflatoxins with variable efficacy depending on the smectite quality. 96% of the ionic interactions between smectite and aflatoxin occurs via two carbonyl oxygens of two planar cycles (D and E) among the 5 of AFB1, and exchangeable cations. However, smectites have very limited adsorption of other mycotoxins than aflatoxins. The mycotoxins adsorption spectrum of smectites can be improved by increasing their interlayer space (d-spacing).
New interactive tool
Olmix has developed a material based on smectite and algae extracts which increases the interlayer space of smectite from 0,7 to 5 nm and thus provides enough space for mycotoxins with voluminous conformations like deoxynivalenol and fumonisins to be adsorbed. This material showed efficacy against a wide range of mycotoxins in a dynamic model as well as many in vivo models without altering nutrients availability. Olmix is also launching a new interactive tool: Myco’Simulator App. The App, available on demand at contact@olmix.com, is a tool to visualise mycotoxin complex conformations and interactions.
Authors: Maria Matard-Mann, Research Project manager and Julia Laurain, For Feed Product Specialist, Olmix France
References available on request



















