Diagnosis of IBV field challenge
Diagnosis of disease can be accomplished by the combined evaluation of flock history, clinical signs, serology and isolation and identification of the pathogen. Serological monitoring can be particularly helpful in establishing the timing and nature of a field infection.
By Dr Bart van Leerdam, BioChek bv, Reeuwijk, the Netherlands, and Dr Pieter Kuhne, Intervet/Schering-Plough Animal Health, Boxmeer, the Netherlands
For unvaccinated flocks, the presence of IBV field challenge can simply be demonstrated by detecting positive IBV serology. However, for IBV vaccinated flocks, identification of a field challenge is more complicated and will require the knowledge of expected vaccination titers (baselines). Every user of an Elisa system should develop his or her own vaccination baselines depending on placement programmes, specific vaccines used and type of birds examined. These factors, in combination with periodic flock profiling, can establish a serological history in order to determine if serological results are normal or abnormal. Unexpected rising titers, significantly higher than the expected vaccination titers, may then indicate the presence of a field challenge. It should be stressed, however, that titers by themselves cannot be used to establish diagnosis. Diagnosis can only be established when one combines serology with clinical symptoms and isolation of the pathogen. Until true diagnosis is established, it is recommended to refer to titers as “suspect of infection” rather then “infection titers”.
Identifying an IBV infection
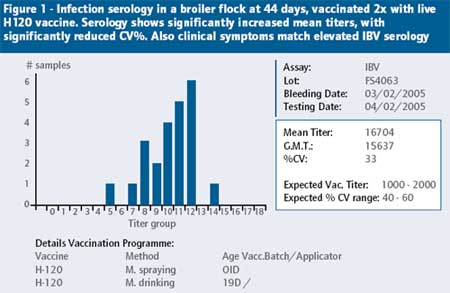
When looking at abnormally high IBV titers, there are three key criteria that have to be met in order to conclude that the serology obtained is the result of an IBV challenge.
1. The mean titer post-infection should be significantly increased. A good rule is that the mean titer post infection must be at least 2x the level you expect after vaccination, or be at least 2x the mean titer level before the infection.
2. The % CV should be significantly reduced. The Coefficient of Variation (CV) should be significantly below levels you expect after vaccination, or significantly below the CV levels before the infection. For instance, if you vaccinate broilers 2x with H120, the expected % CV is between 50-80%. If after vaccination you get a CV of 35%, it is an indication of an abnormal response.
3. The clinical signs must match the serology. If you have elevated IBV serology, but no clinical signs, or signs that do not match IBV infection, you simply cannot confirm an IBV infection.
Figure 1 shows serology of a field case of IBV infection in a broiler flock and helps to illustrate the key criteria mentioned above.
Complicated IBV infections
Although one may suspect an IBV infection when respiratory signs occur, it is advisable to test infected birds against other possible respiratory pathogens as well. The reason for this is 2-fold:
Firstly, IBV infections often occur in combination with other respiratory pathogens such as Avian Rhinotracheitis (ART) (also known as Avian Pneumo Virus, or APV), Ornithobacterium Rhinotracheale (OR), Mycoplasma Gallisepticum (MG), and Mycoplasma Synoviae (MS). Only by looking at the whole serological picture can you diagnose complicated infections. Testing for IBV alone will not show interacting pathogens of respiratory disease.
Secondly, serology of respiratory vaccines such as IBV and NDV are often abnormally affected by lesions present after respiratory challenge (tracheitis and/or airsacculitis). These lesions allow more intensive contact and penetration of the vaccine virus at receptor sites. This results in higher than normal serology after live IBV and NDV vaccination.
This is one of the main reasons why ART infections are often misdiagnosed for IBV infections, looking at IBV serology alone. Looking at the complete serological picture, one can see the interactive patterns with other respiratory disease agents, leading to a correct diagnose of complicated infections. Additionally, the adherence to the key criteria to determine infections (as described above) often helps to rule out “tracheal lesion effect”. In general, enhanced vaccine reactions do not show a doubling of expected mean titers and/or a significant reduction in % CV.
Looking at case histories
The following two case histories are presented to demonstrate the usefulness of full complimentary testing after birds have undergone respiratory disease.
Case history 1:
Chronic respiratory infection misdiagnosed as IBV
A broiler flock was vaccinated twice with live IBV vaccine MA5. At 21 days the birds displayed severe respiratory distress, combined with swollen heads and 10-15% mortality. Post-mortem revealed Airsacculitis with thick yellow foamy exudates, which was attributed to E. Coli infection.
Routine IBV Elisa serology showed slightly elevated mean titers with more uniform response than normal. Figure 2 shows the IBV serology at 43 days of age. Because titers were elevated, it was concluded that the broilers suffered a respiratory challenge with IBV with a secondary infection of E. Coli. A third vaccination of IBV was introduced, but the respiratory signs remained. If a more complete serological investigation had been done, it would have shown significant positive titers for ART and OR (Figure 3a and 3b).
Now looking at the complete serological picture the conclusion on what happened to the flock is different than before. Since the birds were not vaccinated for ART or OR, it can be concluded that the birds suffered a primary infection with ART (tracheitis and swollen heads) and a secondary infection with OR (mortality and cheesy Air sacculitis). The IBV serology does not meet the key criteria for infection, as the mean titers were not significantly (2x) elevated. The slight increase in mean titer and decrease in % CV after vaccination was probably due to tracheal lesion effect caused by the respiratory challenge of ART and OR. The vaccination programme was adapted to include a live ART vaccination at 7 days, and the production parameters returned to normal thereafter.
Case History 2:
Complicated IBV infection with nephritic variant strain
A broiler flock was 2x live vaccinated with live Massachusetts (H120) at 1 and 20 days of age. The birds were vaccinated against NDV 2x with live Avinew at 1 (spray) and 20 days (drinking water). At 14-21 days of age the birds showed respiratory signs, mild diarrhoea and mortality. During post-mortem examination Tracheitis and Nephritis were seen. Some birds were diagnosed as “Cheese Chickens” (Airsacculitis containing thick yellow foamy exudates). The photos illustrate the clinical situation on the farm. Shown are severe tracheitis (Photo 1), inflammation of kidneys (nephritis) (Photo 2) and airsacculitis with thick cheesy exudates (Photo 3).
Figures 4 a-d show the Elisa serology results at 46 days of age. Both MG and MS tested negative (not shown). The IBV serology was abnormally high, with CV (25%) far below expected range (50 – 100%). Also, clinical symptoms matched with the elevated IBV titers.
Serology for ART and OR was also positive, indicating concurrent infections for these diseases, as birds were not vaccinated. The serology for NDV was higher then normal, but the enhanced response was probably due to the “tracheal lesion effect” from the vaccine virus. Also, for NDV the key criteria for infection were not met (mean titer less then 2x expected mean titer level after vaccination). From these results it was concluded that the birds were primarily infected with a nephropathogenic IBV strain, with ART and OR acting as secondary pathogens.
A contingency plan was made, which included a live variant strain IBV (4/91) vaccination at 14 days of age through the drinking water. After introduction of the new programme the production returned to normal. The same serum samples of affected flocks, used for the Elisa were sent to the Intervet service laboratory in Boxmeer, the Netherlands, for serotyping using the Virus Neutralisation (VN) test. Results are summarised in Table 1.
The specific VN test showed the highest titer for the QX-like (or D388) strain the birds were vaccinated with Massachusetts H120 vaccine strain (indicated by VN M41). It can be concluded that the ELISA serology was very helpful in providing an early diagnosis on the nature of the primary disease pathogen (nephritic IBV) and helped to prevent further damage by immediately changing the vaccination programme, which included the variant IBV 4/91 vaccine.
The further serotyping with the VN test helped to establish the final diagnosis to a strain specific level (D338 like strain). It justified the use of a variant strain in the vaccination programme to broaden the IBV protection. The knowledge of the presence of this serotype on this farm can also be helpful to design effective future vaccination programmes for other affected farms in the region.
Conclusions
The main benefits of IBV ELISA serological monitoring to poultry producers are improvement of vaccine application techniques and performance of vaccines, as well as immediate diagnose of disease to identify cause of production losses. The key point of using a monitoring programme is to act on results. Taking immediate appropriate action is crucial to limit and prevent further economic damage to a production. If vaccination results are poor, it allows you to re-evaluate IBV vaccination procedures, and take corrective action. This makes regular monitoring a cost-effective preventative tool. Improving the effectiveness of vaccine application, will result in improved disease control and economic performance of poultry flocks.













