Complex causes need broad prevention and treatment
Various predisposing factors are required to cause clinical and subclinical Necrotic Enteritis. Controlling these factors will, up to a large extend, reduce economic losses.
By Maja Marien, Sharon Miller, Mark LaVorgna, Wouter Depondt and Dieter Vancraeynest, Technical Team Alpharma, EMEA and US
It is generally accepted that predisposing factors are required for Clostridium perfringens to cause Necrotic Enteritis (NE). The best known predisposing factor is mucosal damage caused by coccidiosis, in particular by Eimeria species that colonise the small intestine (E. maxima, E. mitis, E. acervulina).
Coccidiosis often proceeds or occurs concurrent with field outbreaks of NE. Eimeria parasites destroy intestinal cells, causing gaps in the intestinal lining through which plasma proteins leak into the gut lumen (Figure 1). Furthermore, coccidiosis causes an impaired intestinal function resulting in poorly digested feed remaining in the intestine, as well as an inflammatory response that leads to increased mucus production. All of these – the plasma proteins, poorly digested feed and mucus – are suitable as growth-substrate for extensive proliferation of clostridial bacteria and hence support bacterial overgrowth.
Effect of the diet
The nature of the diet is an important non-bacterial factor that influences the incidence of NE. Anything that increases digesta viscosity, decreases nutrient digestibility and prolongs intestinal transit time, can be considered as a risk factor. More nutrients available in the gut can stimulate the growth of certain bacterial populations, which in its turn might change the general intestinal microbial composition. It has been shown that diets with high levels of non-starch polysaccharides (NSPs) increasing the viscosity, high dietary concentrations of animal protein (e.g. fishmeal) and/or protein-rich diets containing relatively high concentrations of poorly digestible proteins can result in heavily increased proliferation of C. perfringens resulting in clinical problems.
Apart from Eimeria infections and the feed, any factor that causes stress (e.g. increased stocking density) in broilers could predispose them to NE because it could alter the intestinal environment.
Mortality rates
In the acute form of NE, the prominent feature is acute death, often without premonitory signs. Mortality rates can vary from 5-50%. Rates of about 10% are generally not unusual. Other clinical signs of NE are aspecific and include depression, dehydration, somnolence, ruffled feathers, diarrhoea, increased water, and decreased feed consumption. Wet litter can also be an early indicator of disease.
At necropsy, the small intestine is thin-walled, fragile, dilated, fibrinonecrotic and filled with gas. Confluent or focal mucosal necrosis of the small intestine covered by a yellow-brown or bilestained pseudomembrane can be found. Although NE is of importance worldwide, there are intestinal conditions that are not necessarily caused by C. perfringens, which are of even bigger concern to poultry producers. As these conditions have many resemblances with NE regarding predisposing factors, prevention and treatment, they are discussed in more detail below.
Subclinical NE or dysbacteriosis?
A subclinical form of NE is also described in literature. In this form, C. perfringens causes chronic damage to the intestinal mucosa, which leads to decreased digestion and absorption, reduced weight gain, and increased FCR, but without associated mortality.
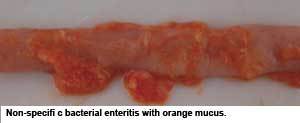
Non-specific bacterial enteritis (also referred to as dysbacteriosis; small intestinal bacterial overgrowth, or SIBO) seems to be yet another form of bacterial enteritis. At least under European production conditions (without growth promoters and meat- and-bone meal), this non-specific bacterial enteritis is of far higher importance than NE, with estimates of 50% of the flocks being treated for this condition. In some cases, when broilers face nonspecific bacterial enteritis, an overgrowth of C. perfringens in the small intestine is encountered. Although C. perfringens is attributed, by many, to have a significant role in the condition, some believe C. perfringens overgrowth is a secondary finding rather than a primary cause. The general consensus is that non-specific bacterial enteritis is due to a disruption of the normal microbiota with an overgrowth of mainly Gram-positive bacteria (the term “dysbacteriosis” refers to this imbalance in the intestinal microbiota). The reason why it is believed to involve mainly Gram-positive bacteria is because antibacterial agents with a Gram-positive spectrum are successfully used to treat the clinical signs, which include wet litter, diarrhoea, feed intake remaining stagnant for a couple of days, and increased water/feed ratio. At necropsy, the intestinal wall appears fragile, thin and lacks sufficient tonus. Excessive watery contents throughout the small intestine, ballooning of the intestine, undigested feed particles in the caudal part of the intestine, an oily, greasy layer on top of the feed, as well as orange mucus in the intestinal lumen, are also indicative. Sometimes inflammation and/or hyperemia of the intestine are obvious. The negative effect on performance is also the primary concern here. There is still scientific debate about the difference between non-specific bacterial enteritis and subclinical NE, as both conditions resemble each other on necropsy. Predisposing factors for non-specific enteritis are largely the same as for (subclinical) NE. The most important known predisposing factor is intestinal damage caused by coccidia.
Prevention and treatment
Looking at the pathogenesis of NE and non-specific bacterial enteritis, it goes without saying that control of predisposing factors will be of utmost importance. As a start, optimal control of coccidiosis, also of subclinical forms, is essential. Rotation programmes are a key element for long-term coccidiosis prevention. Rotation is based on timely changes from one in-feed anticoccidial to one of another class (ionophore or chemical; see Table 1). This will prevent the development of resistance in the Eimeria population and therefore preserve long-term anticoccidial efficacy (for further details, see World PoultryCoccidiosis Special, 2008). Furthermore, optimising the feed formulation (good quality proteins and fats, lower level of NSPs by using appropriate enzymes) will definitely prove to be beneficial.
In North America, factors contributing to the lower incidence of NE include corn-based diets that often contain animal by-products that do not favour growth of C. perfringens, as well as the use of in-feed medications such as BMD® (Alpharma) that are approved for the control and prevention of NE caused by C. perfringens. European farmers rely mostly on curative use of antimicrobials with a Gram-positive spectrum (e.g. amoxicillin, penicillin, lincomycin, tylosin) to control NE whenever clinical signs become apparent. Amoxicillin and penicillin are often preferred because of their very good and rapid action (drastic improvement within 24 hours).
Additionally, in cases of severe non-specific bacterial enteritis these same antimicrobials are often used. Next to that, increasingly more interest is seen towards alternative non-antibiotic approaches to control NE and non-specific bacterial enteritis. The knowledge on the use of these products is increasing daily, and it becomes clear that these alternatives can definitely play a role, albeit mainly in the prevention of problems. One such alternative product, ProFlora™ (Alpharma), a combination of a direct fed microbial (Bacillus subtilis QST 713) and a probiotic (β-glucans + β-mannans), has demonstrated efficacy in the control of NE caused by C. perfringens in broilers. ProFlora™ aims at re-establishing the natural gut flora often disrupted by the conditions previously discussed. The β-glucan and β-mannan fraction has been shown to stimulate the immune system, and improve FCR and weight gain by significantly enhancing gut maturation. Other disease control strategies are also looked into, like, the development of vaccines (e.g. vaccines using recombinant alpha-toxin) in order to stimulate protective antibodies. Very recently the concept of using bacteriocins active against C. perfringens are investigated as a possible alternative to control NE.













