Histomoniasis in turkeys: Challenges, opportunities and solutions

Histomoniasis, also known as blackhead, was identified as a parasitic disease of gallinaceous birds over 130 years ago when it was first reported in a turkey flock in Rhode Island, USA. Unfortunately, it still negatively affects poultry health today. This article explores the disease dynamics, developments and solutions.
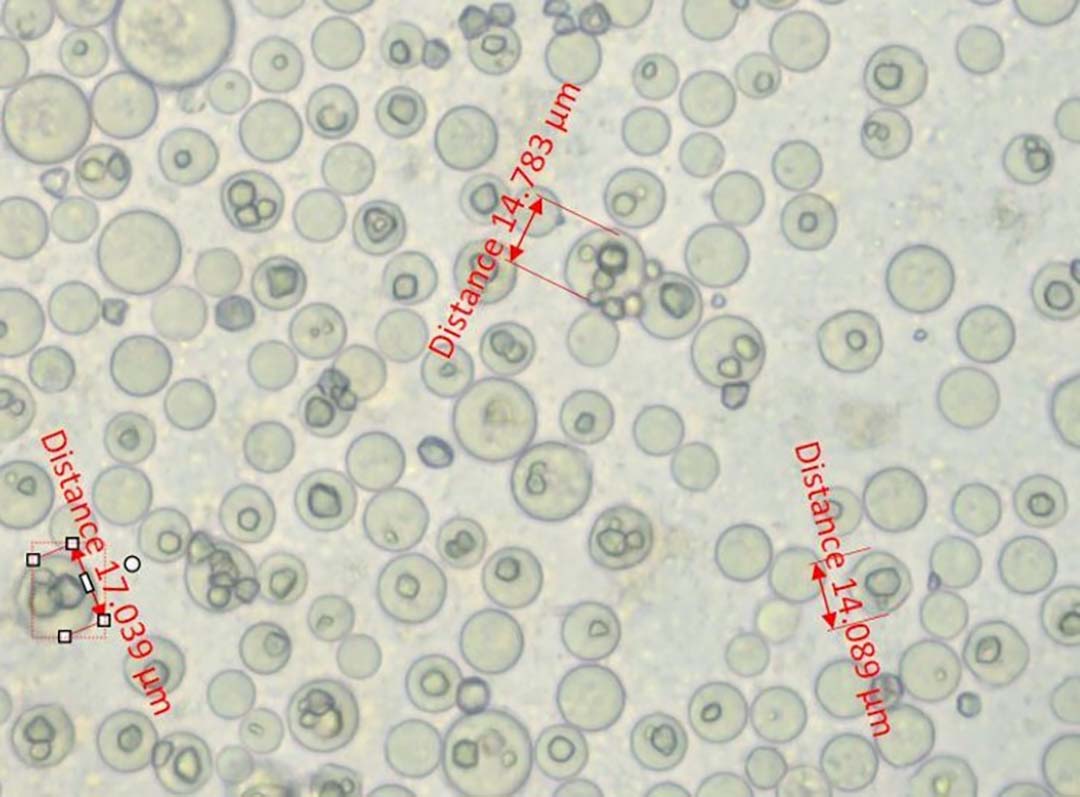
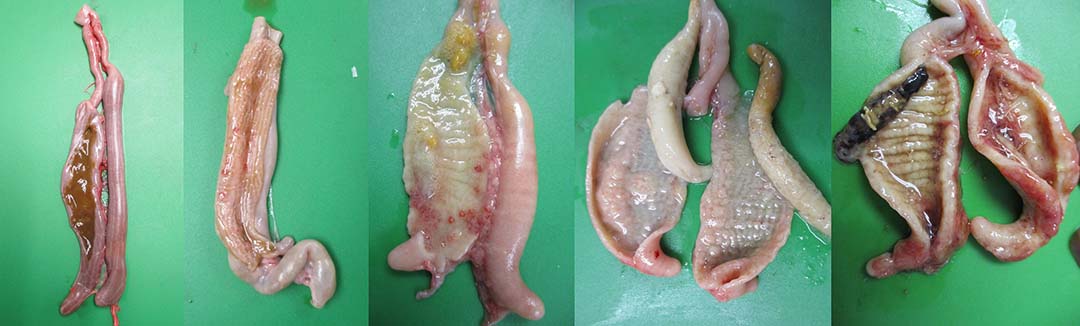
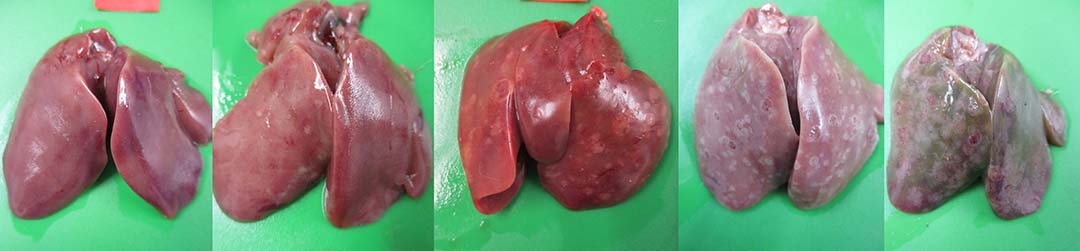
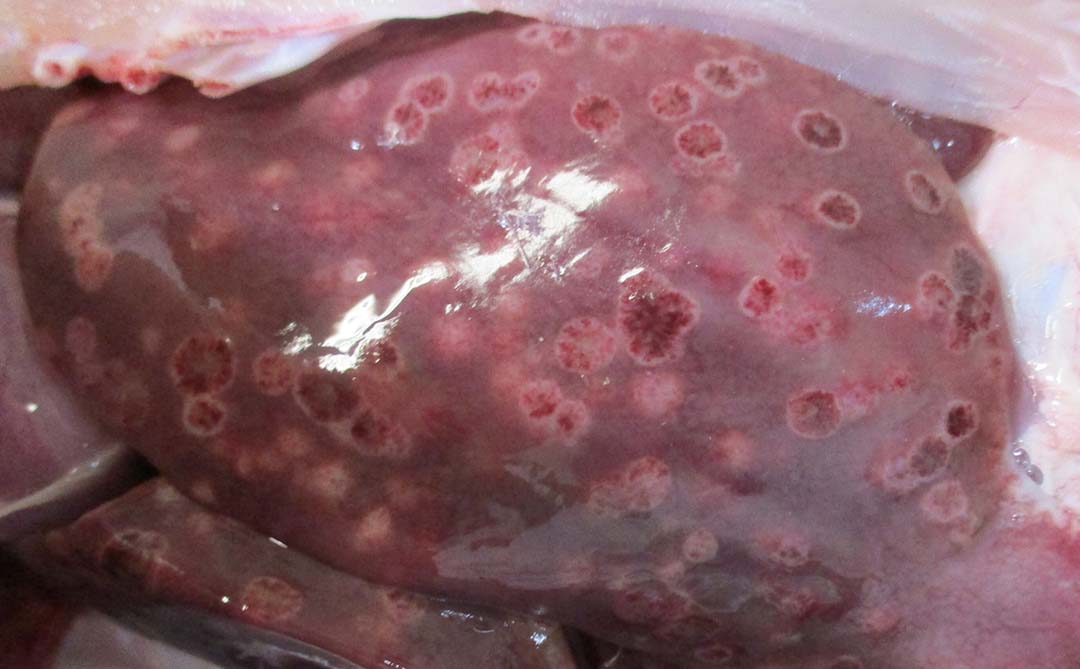
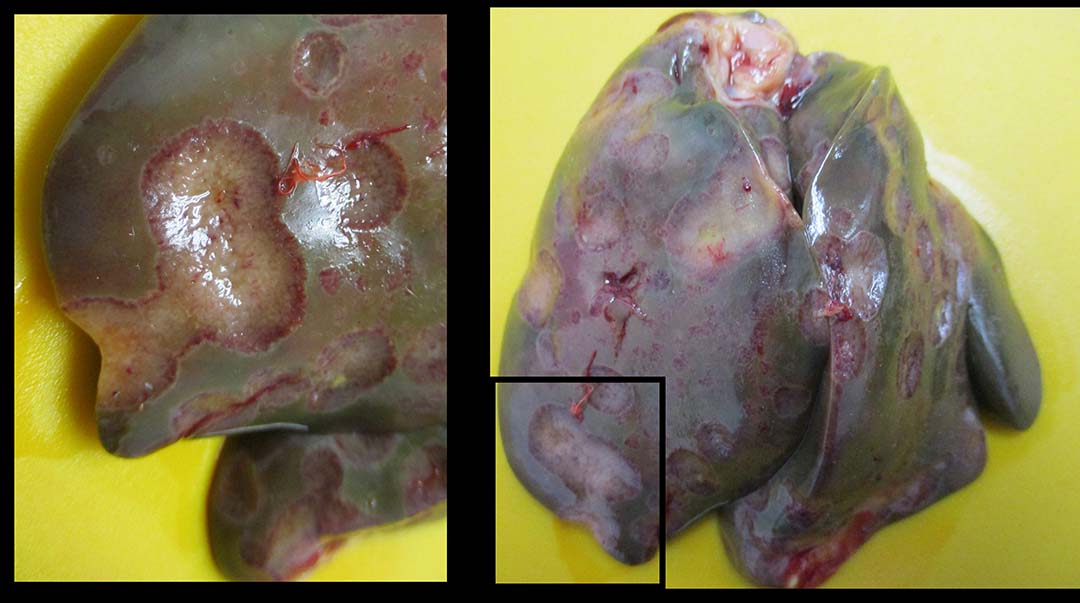
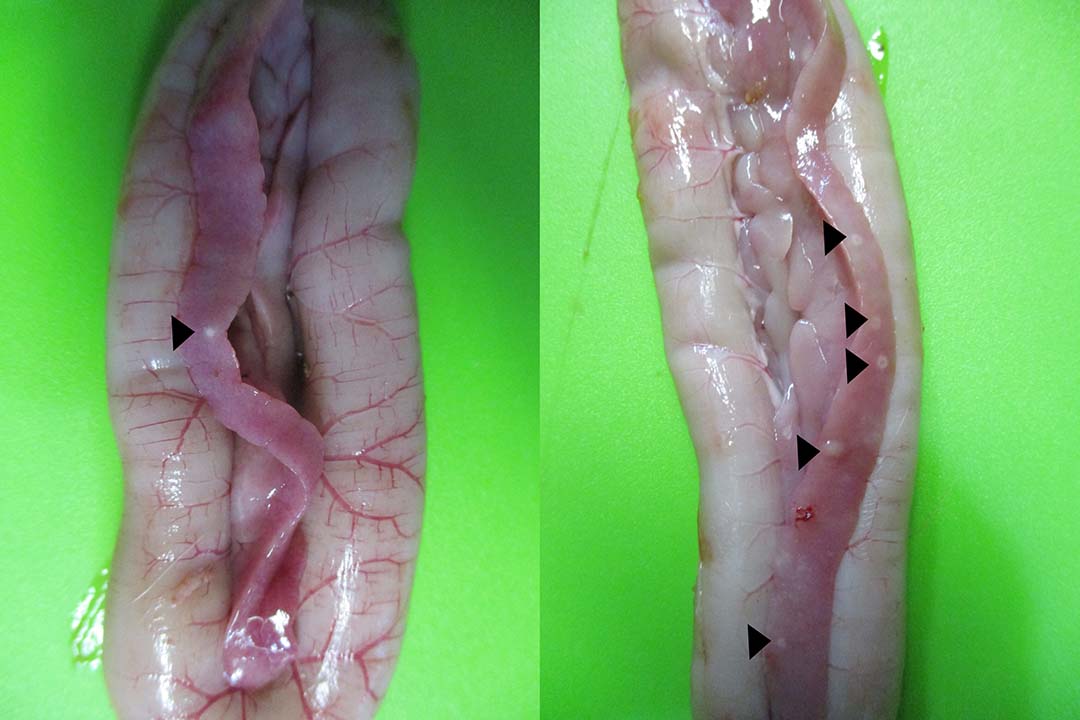
Histomoniasis, caused by an extra-cellular protozoan parasite Histomonas meleagridis (Figure 1), is considered a fatal disease in turkeys with mortalities of up to 80-100%. H. meleagridis targets the ceca and causes inflammation, resulting in the formation of cecal core and necrosis (Figure 2). H. meleagridis enters the hepatic portal vein to the liver, the other target organ. In the liver, it causes white, tan, pink or red necrotic spots (Figure 3), lesions appearing as dark spots surrounded by a peripheral circle resembling a ‘bulls-eye’ (Figure 4), or shallow depressions resembling saucer-shaped lesions (Figure 5).
Typhilitis with cecal core and necrotic spots in the liver are considered the hallmark lesions of histomoniasis. Apart from H. meleagridis, Tetratrichomonas gallinarum and Salmonella Arizonae cause lesions in both the liver and ceca of turkeys. The systemic spread of H. meleagridis results in necrotic spots in other visceral organs, such as the spleen, kidney and pancreas (Figure 6).
Histomoniasis causes general clinical signs which include, but are not limited to, dullness, depression, anorexia, a hunched up stance, huddling, ruffled feathers and droopy wings (Figure 7). Histomoniasis produces yellowish-green diarrhoea or sulphur-yellow diarrhoea (Figure 8) and, occasionally, blood flecks may be noticed.
Clinical signs are usually seen around 1 week after infection, with mortality proceeding in the following week. Consequently, the interval between the appearance of clinical signs and mortality is minimal. Thus, by the time the clinical signs appear, a significant amount of damage has already occurred in the ceca and liver, compromising function and impairing systemic health.
How is H. meleagridis transmitted to a turkey flock?
A lack of appropriate biosecurity measures allows H. meleagridis to be easily transmitted from an infected flock to a healthy flock of birds. Turkeys resting on litter contaminated with H. meleagridis enables the pathogen to enter the cloaca and reach the ceca target organ, thereby inducing infection.
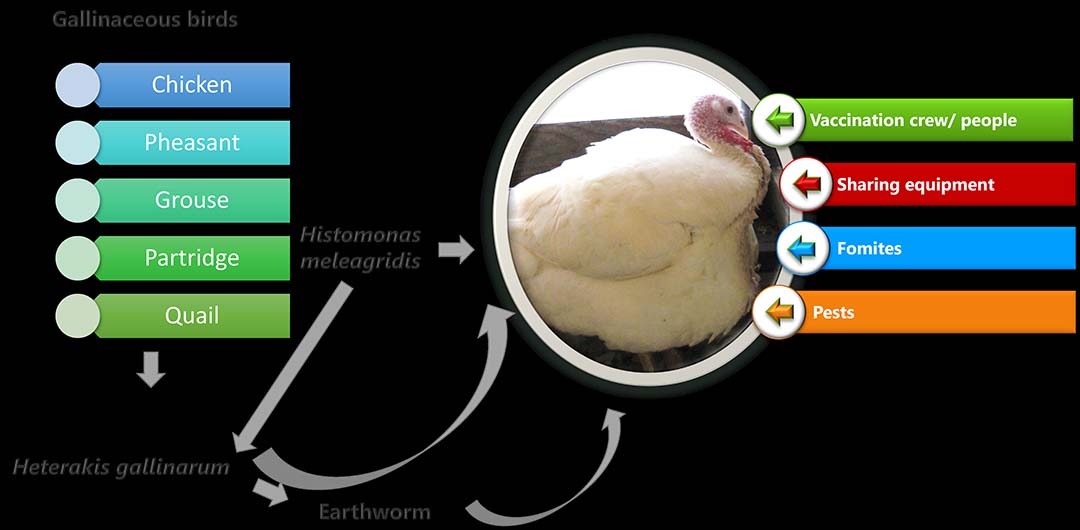
Heterakis gallinarum, the cecal nematode of gallinaceous birds, is an intermediate host and considered a potential carrier of H. meleagridis. Most H. gallinarum eggs are found to be infected with H. meleagridis. Fomites, arthropod vectors, flies, equipment, vaccination crew or anyone entering the barn can harbour the eggs of H. gallinarum and cause transmission of the disease.
Earthworms consuming the eggs of H. gallinarum act as a paratenic host and serve as a carrier for H. meleagridis. Turkeys consuming the eggs or earthworms harbouring the eggs of H. gallinarum infected with H. meleagridis can be infected with histomoniasis (Figure 8).
How is H. meleagridis disseminated within the flock?
H. meleagridis affects the intestinal health of turkeys resulting in diarrhoea which indirectly increases the moisture load of the litter. The infected turkeys shed H. meleagridis in their faeces, which further facilitates the spread of histomoniasis within the flock. H. meleagridis can survive in faeces for 9 hours.
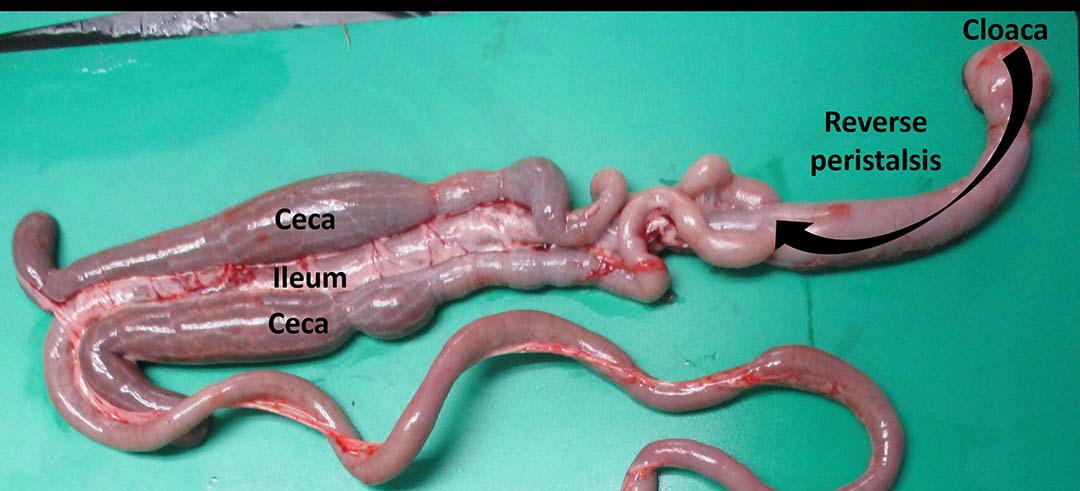
Histomoniasis can be transmitted between turkeys through faeces containing H. meleagridis. It is well documented that histomoniasis is transmitted between the birds by the cloacal drinking phenomenon exhibited by turkeys. During cloacal drinking, the reverse peristalsis movement of the intestine favours the movement of H. meleagridis from the contaminated litter enabling it to enter through the cloaca and reach the ceca (Figure 9).
Coprophagy (litter pecking behaviour) and faecal-oral transmission also contributes to the spread of histomoniasis within the flock.
Why are turkeys severely affected by H. meleagridis?
Compared with chickens, turkeys lack a competent cecal innate immune response and fail to elicit an effective immune response to block H. meleagridis at the cecal level. As a result, H. meleagridis can enter the hepatic portal vein and reach the liver, resulting in liver degeneration and necrosis, eventually leading to mortality.
In addition, turkeys are over-reactive and generate immoderate immune responses due to hyper-inflammation. This response induces a cytokine storm which severely damages the visceral organs, resulting in mortality.
When do histomoniasis outbreaks occur?
Histomoniasis outbreaks have been documented throughout the year but increased incidences have been reported during the summer and rainy seasons. Heat stress has a negative impact on intestinal integrity and the microbiome which may increase the risk of H. meleagridis invading the ceca and inducing disease. On rainy days earthworms and other arthropod vectors seek a warm shelter.
The temperature of turkey barns is conducive to that which will attract worms and arthropod vectors. If the turkey barn does not have a strict biosecurity plan and effective pest control programme in place, then these pests can easily enter the barn resulting in histomoniasis outbreaks.
Although histomoniasis outbreaks are reported in all age groups, more incidences have been documented around 6-8 weeks of age. As turkeys are moved from the brooder barn to the grower barn at around 6-7 weeks of age, there is more chance of them coming into contact with H. meleagridis at this time.

How was histomoniasis controlled in the past?
Substantial prophylactic and therapeutic measures using nitro-imidazoles (dimetridazole, ipronidazole), nitrofurans (furazolidone, salfuride) and arsenics (nitarsone) have kept histomoniasis under control for decades.
Dimetridazole was banned in the US (1987) and EU (1995, 2001) and ipronidazole was withdrawn/banned in the US (1989) due to concerns about toxic residues. Furazolidone was banned in the US (1991) and salfuride was banned in the EU (2002-2003), thus ending the chapter of nitrofurans. Nitarsone was withdrawn in the US and Canada (2016).
Despite the ban and withdrawal of prophylactic and therapeutic products as a result of regulatory action, increased field outbreaks of histomoniasis have been reported across the globe. As a result, calls to identify solutions to deal with histomoniasis have intensified, with a need to evaluate various prophylactic and therapeutic compounds.

What are the challenges?
After the withdrawal/ban of prophylactic and therapeutic treatment products for histomoniasis, several intervention measures have been evaluated by various labs in universities, as well as biological, pharmaceutical and animal health companies, to find a solution to histomoniasis.
In recent years, several chemical compounds and phytochemicals have been tested against H. meleagridis in turkeys but were not found to be effective. Thus, identifying a compound for the prophylactic/therapeutic treatment of histomoniasis has been elusive.
Live attenuated vaccines have been developed by several research labs worldwide. However, the attenuated vaccines are not all the same and some vaccines have been found to confer protection against heterologous challenge, while others do not. Attenuated vaccines developed by research labs have not so far been commercially available globally.
Like viral or cell-culture cultivation in the lab, H. meleagridis cannot be grown in a sterile media. H. meleagridis is grown in a specialised media like Dwyer’s media or modified Dwyer’s media which contains Medium 199 with Hanks balanced salt solution, sodium bicarbonate, horse serum and rice powder. In addition, H. meleagridis cannot be cultured alone and needs to be cultured along with bacteria which provide nutrients for H. meleagridis.
Opportunities
Although strict biosecurity measures and enhanced management conditions can prevent the spread and transmission of histomoniasis, the poultry industry cannot rely exclusively on these measures to prevent histomoniasis. The need to identify prophylactic and therapeutic treatments to combat histomoniasis therefore cannot be understated. Identifying a solution to histomoniasis is a formidable task facing the turkey industry.
Currently, there are no commercial vaccines available to prevent histomoniasis. With no alternative prophylactic/therapeutic treatments globally available to prevent or treat this deadly disease it is imperative that solutions to histomoniasis are identified. There are ample research opportunities to study the risk and virulence factors associated with histomoniasis outbreaks.
From the prophylactic perspective, novel commercial vaccines are needed for the global turkey industry. From the prophylactic/therapeutic perspective, new chemical compounds or phytogenic products are needed. Animal health companies and universities can seize this great opportunity by identifying and implementing a prophylactic or therapeutic strategy that will yield a fruitful solution to histomoniasis.
Solutions
A global solution to histomoniasis is not yet in place. Certain antibiotics and phytogenic products for histomoniasis are available in a few geographical regions but not on a global scale. Under the current scenario, a holistic approach through taking precautionary steps, can help to reduce the incidence of histomoniasis.
1. Gut health: Preventing H. meleagridis at the target site is an effective way to combat histomoniasis. Maintaining gut integrity is the best way to minimise the risk of histomoniasis.
2. Concurrent infections: Concurrent infection of H. meleagridis along with other infectious pathogens, such as Eimeria species, E. coli, Salmonella, Clostridium and hemorrhagic enteric virus can worsen and exacerbate mortality and morbidity. Although a global solution to histomoniasis is currently unavailable, prophylactic solutions are available for the concurrent infectious pathogens. Using prophylactic measures for other pathogens reduces the mortality and morbidity associated with histomoniasis.
3. Pest control programme: Adopting a strategic pest control programme limits the risk of histomoniasis disease transmission. The eggs of H. gallinarum can easily adhere to arthropod vectors, such as darkling beetles and aid in the spread of histomoniasis. Flies also play a role in transmitting histomoniasis. H. gallinarum eggs are very resistant to environmental conditions and commonly used disinfectants. In addition, H. gallinarum eggs can survive for several years. An effective pest control programme plays a vital role in minimising the risk of spreading histomoniasis between flocks.
4. Litter management: H. meleagridis can survive in the litter for several hours and high moisture levels favour its survival. H. meleagridis is highly susceptible to environmental conditions and commonly used disinfectants. Thus, proper litter management is advisable to reduce the moisture level and lower the burden of H. meleagridis in the litter. Many litter treatment products, such as lime, salt and copper sulphate, are available on the market. If the litter is wet due to loose droppings, remove the wet litter or top dress it with fresh litter. If there are any water spills, these should be addressed immediately.
5. Migration fences/partitions: Infected birds need to be identified based on clinical signs, such as sulphur-yellow faeces and yellow staining of feathers around the vent region. The affected birds then need to be necropsied to confirm the gross lesions of histomoniasis. If the infected birds are identified only in certain areas of the barn, then a partition will help to prevent disease transmission between the birds. If the infected birds are spread throughout the barn, then a partition may not help to prevent the transmission of the disease.
6. Removal of dead birds: Dead birds left in the barn for a long time increases the risk of disease transmission to adjacent birds. Thus, picking up dead birds at regular intervals, at least 2-3 times a day, is advisable. Dead birds need to be disposed of properly.
7. Feed management, quality and mycotoxins: Avoid feed outages as this can adversely affect the integrity of the intestine and the microbiota. Poor quality feed and mycotoxins attack the integrity of the gut. Any inadvertent damage to the gut provides a niche environment that will favour H. meleagridis infection.
8. Bird density: Adhere to the bird density requirements as per standard recommendations. Overcrowding adversely affects the intestinal microbiome and its integrity.
9. Effective downtime: At least a two-week period of downtime is recommended between turkey placements.
10. Ventilation: Adequate ventilation measures need to be implemented to avoid high relative humidity in the barn.
11. Stress factors: Stress factors can originate from many sources, such as feed quality, mycotoxins, bird movement, density, infectious pathogens, improper management or an ineffective pest control programme. Minimising stress factors will maximise the success rate in combatting histomoniasis.
12. Biosecurity: Observe strict biosecurity measures to prevent histomoniasis. Vehicles entering and leaving the farm premises must be disinfected.
13. Neighbourhood farms: All gallinaceous birds harbour H. gallinarum. In fact, the H. gallinarum worm burden is heavy among long-lived birds, such as broiler breeders and layers. Raising turkeys close to a broiler breeder and layer farm increases the chance of histomoniasis outbreaks. Avoid raising turkeys close to a broiler breeder. In view of H. gallinarum, backyard chickens should also not be ignored.
*Photos and illustrations presented in all the figures are by Vijay Durairaj and Ryan Vander Veen.












